Do I Need to Apply? Determining Exempt, Expedited, or Full Board Review
Studies will qualify for one of three types: Exempt, Expedited, or Full Board. Please utilize the flow chart at the bottom of this page to assist you in determining which category your activities fall under.
Exempt
Certain forms of research are exempt studies that do not need to undergo IRB review. Exempt studies are, for example, those that involve usual educational practices or are not greater than minimal risk. The Department of Health and Human Services’ Office for Protection from Research Risks (OPRR) states that research which is exempt from review by the IRB includes those which fall into at least one of the categories listed below:
- Research conducted in established educational settings that involves usual educational practices;
- Research involving educational tests which are part of an accepted curriculum;
- Surveys, interviews or observations of public behavior where the subject cannot be identified by the collected data;
- Archival research studying existing data, documents or specimens.
- In addition, some research will be eligible for expedited review, if it involves minimal risk to human subjects and is covered under the list of categories of minimal risk published by OPRR.
On the other hand, any research that involves children, or research that probes into sensitive private information (e.g., medical or criminal records) calls for review.
In parallel with protocol review, the IRB serves to advise members of the faculty on their research questions, and when in doubt about whether a particular project falls the exempt category, the researcher should consult with the IRB.
Expedited
Expedited studies are reviewed by a single IRB member (typically the IRB Chair or a reviewer designated by the Chair) and not the entire board. If the single reviewer cannot approve it, then the study will be reviewed by the full board. Expedited studies are not greater than minimal risk and fall into at least one of the exempt categories (listed above).
Full Board
If studies do not qualify for exempt or expedited review or are greater than minimal risk, then the research must submit a protocol application for review by the IRB at the monthly meeting.
Do I need to apply?
The UDC IRB reviews human subjects research, so before applying you need to ask yourself: 1) “Is it research”? and 2) “Are there human subjects involved?”. Using the flow chart below, where do you wind up?
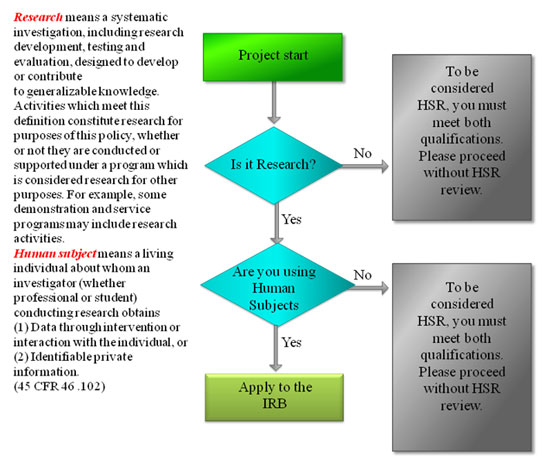
What is an intervention? Intervention includes both physical procedures by which data are gathered (for example, venipuncture) and manipulations of the subject or the subject’s environment that are performed for research purposes. Interaction includes communication or interpersonal contact between investigator and subject. Private information includes:
- Information about behavior that occurs in a context in which an individual can reasonably expect that no observation or recording is taking place, and
- Information which has been provided for specific purposes by an individual and which the individual can reasonably expect will not be made public (for example, a medical record).
Private information must be individually identifiable (i.e., the identity of the subject is or may readily be ascertained by the investigator or associated with the information) in order for obtaining the information to constitute research involving human subjects (45 CFR 46.102)






